Vero Cell Vaccine Sinopharm Or Sinovac - Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use
NASDAQSVA Sinovac or the Company a leading provider of biopharmaceutical products in China announced today that the inactivated. Le produit de Sinopharm est un vaccin inactivé appelé vaccin SARS-CoV-2 Vero Cell.
Who Begins Shipping Chinese Vaccines Despite Some Misgivings Reuters
Sinopharms other vaccine developed via a Wuhan unit and not listed for emergency use by the WHO has an efficacy rate of around 73.

Vero cell vaccine sinopharm or sinovac. Sinopharm is the only vaccine approved in the country. EMAs human medicines committee CHMP has started a rolling review of COVID-19 Vaccine Vero Cell Inactivated developed by Sinovac Life Sciences. Sinovac Biotech Ltd.
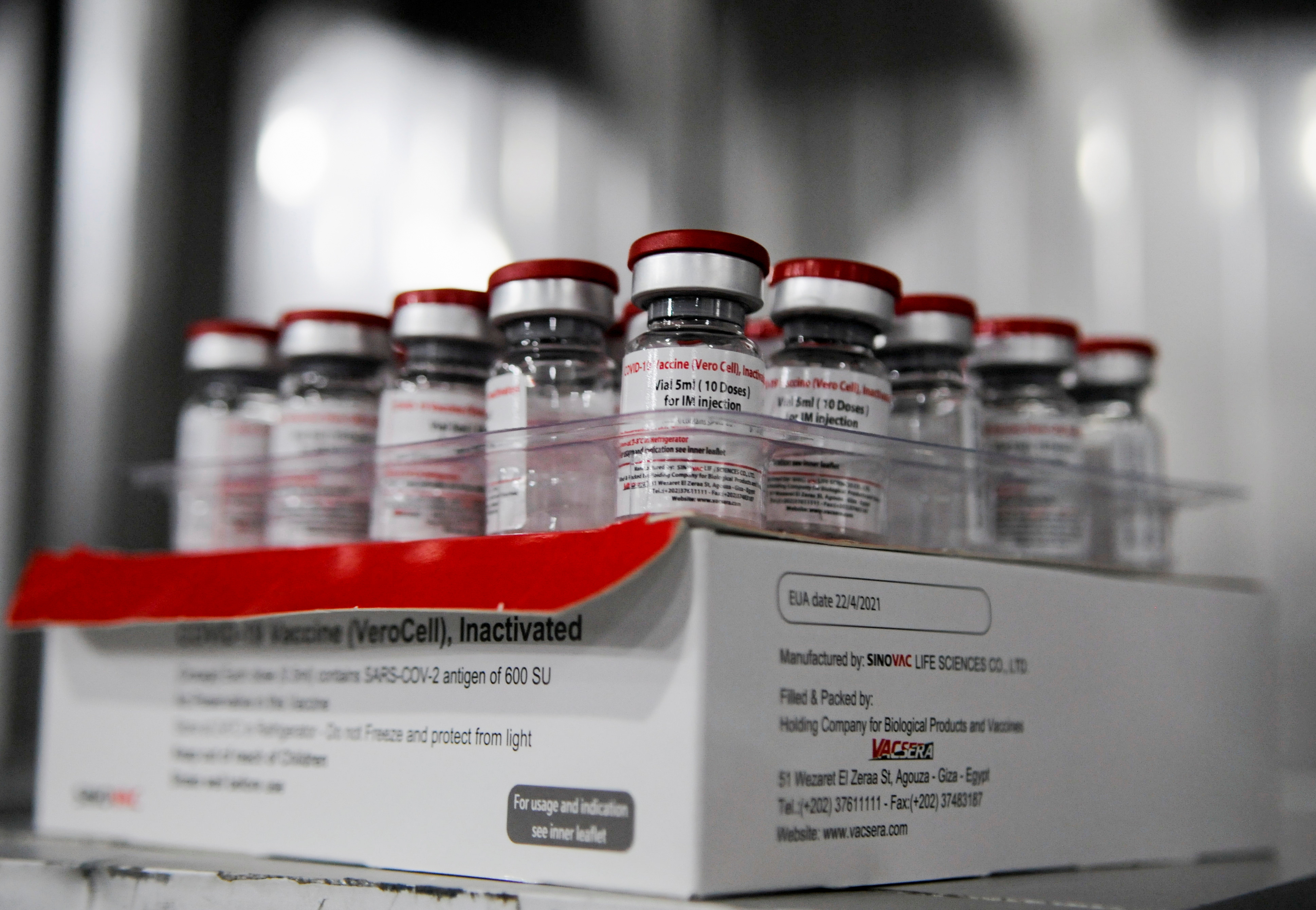
COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. The antibodies attach to viral proteins such as the. BBIBP-CorV also known as the Sinopharm COVID-19 vaccine or BIBP vaccine is one of two inactivated virus COVID-19 vaccines developed by Sinopharms Beijing Institute.
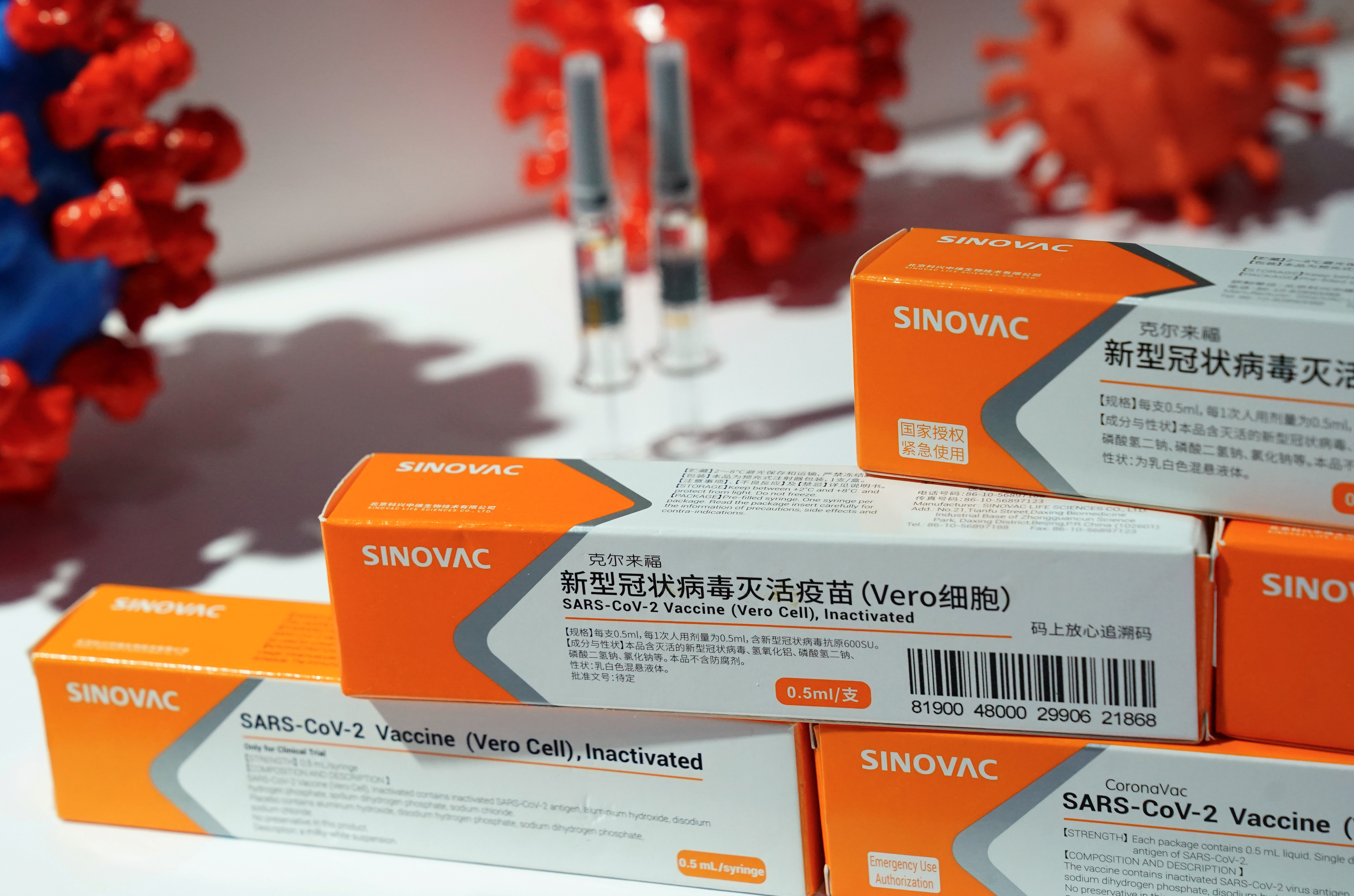
A number of African Latin American and Asian countries have chosen to approve and use the Vero Cell vaccine by the Beijing-based pharmaceutical company Sinovac and. Vero Cell Vaccine Vial Sinovac Vaccine 2nd Chinese Dose Greenlighted By Who More To Follow Global Times - Who today listed the sinopharm covid19 vaccine. Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac.
The EV71 vaccine an innovative vaccine developed by Sinovac against hand foot and mouth disease caused by EV71 was commercialized in China in 2016. Les conditions de stockage peu contraignantes le rendent parfaitement. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements.
The other vaccines approved in the country are RBD-Dimer CanSino SARS-Cov-2 Vaccine Vero. Peer-reviewed results published in JAMA of Phase. SINOPHARMS BBIBP-CorV works by teaching the immune system to make antibodies against the SARS-CoV-2 coronavirus.
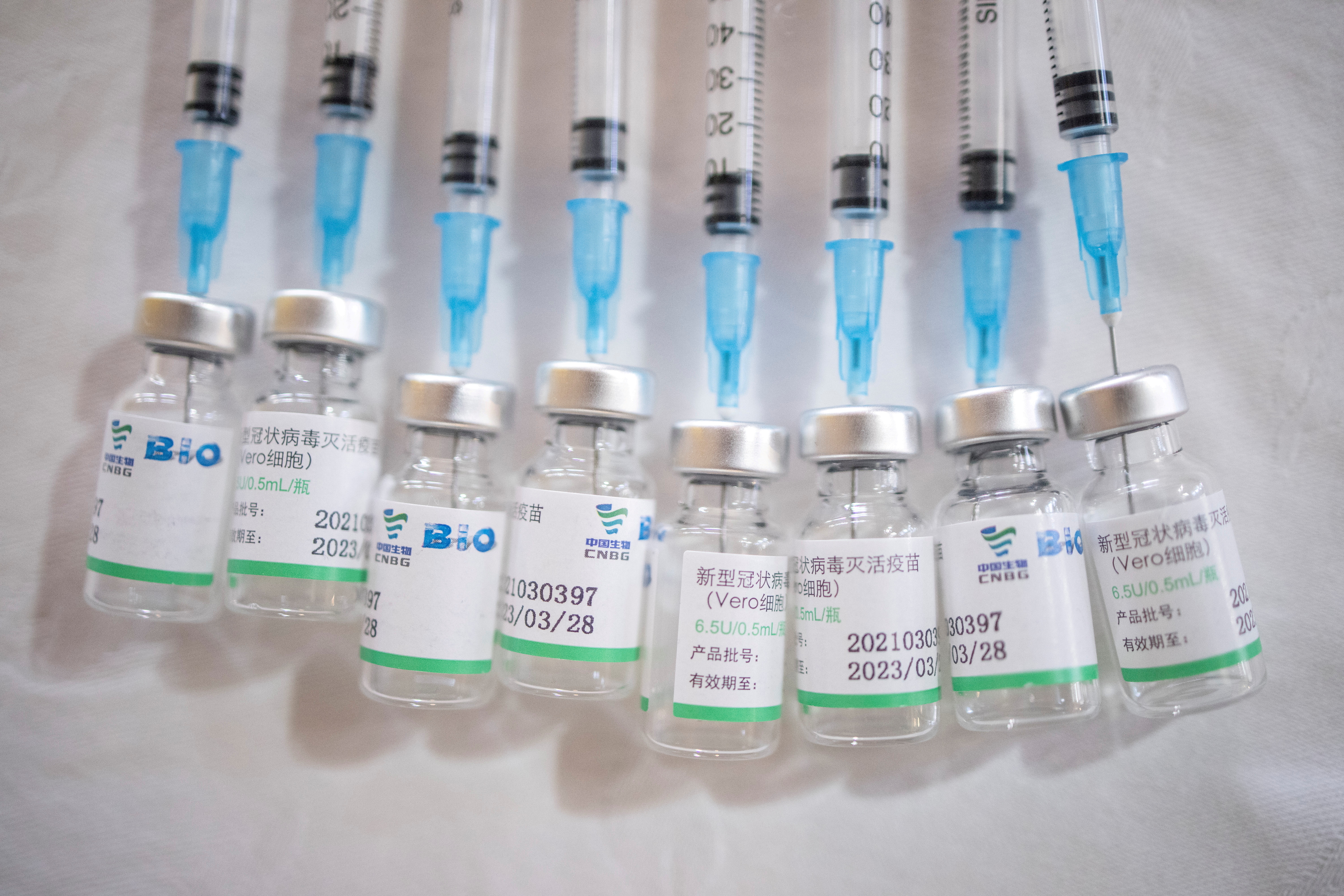
We have now learned that the two Chinese vaccines developed by Sinopharm and Sinovac have also passed their Phase 3 clinical trials with an efficacy of 79 and. SAGE recommends the use of Sinovac-CoronaVac vaccine as 2 doses 05 ml given intramuscularly. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
Its easy storage requirements make it highly suitable for low-resource. WHO recommends an interval of 24 weeks between the first and second. Sinopharm has been reported to have a 79 per cent efficacy rate while results from Sinovac have ranged from 504 per cent to 835 per cent.
Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant. Sinopharm has two vaccines in development one developed by the Wuhan Institute of Biological Products and another by the Beijing Institute of Biological Products. Recommendation for an Emergency Use Listing EUL of SINOVAC Covid-19 vaccine Vero cell Inactivated - CoronaVac TM Published.

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Explainer Are Chinese Covid 19 Shots Effective Against The Delta Variant Reuters

Sinovac And Sinopharm What Do We Know About The Chinese Vaccines Bbc News

Questions And Answers On Sinopharm Covid 19 Vaccine Bangkok Hospital

Who Says Decision On Sinopharm And Sinovac Vaccines To Come Next Week Cgtn

Study Chinese Covid Shot May Offer Elderly Poor Protection

Sinopharm And Sinovac To Deliver 550 Million Vaccine Doses To Gavi

China Vaccine Maker Sinopharm Says Chairman And A Director Resigned

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases

Sinopharm Sinovac To Supply 550m Vaccines To Covax Global Times

Chinese Drugmaker Gives Trial Coronavirus Vaccine To Staff Nikkei Asia

China S Sinovac Phase Iii Trials In Brazil Could Last As Little As Three Months 2020 07 10 Bioworld
Gavi Signs Agreements With Sinopharm And Sinovac For Immediate Supply To Covax Gavi The Vaccine Alliance
Who Sets Dates For Reviews Of Sinopharm And Sinovac Covid 19 Vaccines Reuters

Chinese Covid 19 Vaccine Is 86 Effective Uae Says Time
Who China Chief Says Approval For 1st Chinese Vaccine A Milestone Achievement World News India Tv